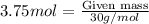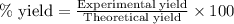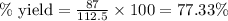Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
Answers: 1

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2
You know the right answer?
The first step in the ostwald process for producing nitric acid is 4nh3(g) + 5o2(g) -> 4no(g) +...
Questions


Mathematics, 06.04.2020 16:59

Mathematics, 06.04.2020 16:59

Physics, 06.04.2020 16:59



Mathematics, 06.04.2020 16:59

Mathematics, 06.04.2020 16:59



Mathematics, 06.04.2020 16:59


English, 06.04.2020 16:59







Computers and Technology, 06.04.2020 16:59

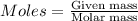
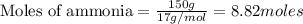
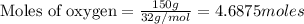
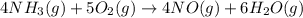
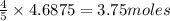 of ammonia
of ammonia