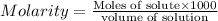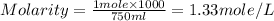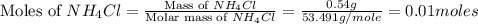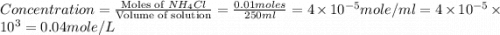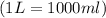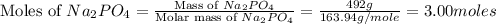Chemistry, 26.06.2019 15:30 genyjoannerubiera
1.distinguish between a 1m solution and a 1m solution. 2. calculate the molarity of 1.0 mol of kcl in 750 ml of solution. 3. what is the concentration (in m) of each of the following solutions? a. 0.54g of ammonium chloride in 250 ml of solution b. 492g of sodium phosphate in 500 ml of solution

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is the molality of a solution that has 4 mol of kci in 0.800 kg of water
Answers: 3

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3
You know the right answer?
1.distinguish between a 1m solution and a 1m solution. 2. calculate the molarity of 1.0 mol of kcl...
Questions

Mathematics, 29.01.2020 21:51

History, 29.01.2020 21:51


History, 29.01.2020 21:51



Mathematics, 29.01.2020 21:51

English, 29.01.2020 21:51





History, 29.01.2020 21:51







History, 29.01.2020 21:51