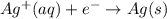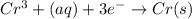Chemistry, 01.07.2019 06:30 trosclairozlynn02
The following equations are half reactions and reduction potentials. ag+ (aq) + e– ag(s) has a reduction potential of +0.80 v. cr3+ (aq) + 3e– cr(s) has a reduction potential of –0.74 v. which statement best compares the substances in these half reactions? a silver ion gains electrons more easily and is a stronger oxidizing agent than a chromium(iii) ion. a silver ion loses electrons more easily and is a stronger oxidizing agent than a chromium(iii) ion. a silver ion gains electrons more easily and is a stronger reducing agent than a chromium(iii) ion. a silver ion loses electrons more easily and is a stronger reducing agent than a chromium(iii) ion. the answer is a

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1


Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3
You know the right answer?
The following equations are half reactions and reduction potentials. ag+ (aq) + e– ag(s) has a reduc...
Questions

Chemistry, 02.09.2019 06:10




English, 02.09.2019 06:10



Biology, 02.09.2019 06:10




Computers and Technology, 02.09.2019 06:10

SAT, 02.09.2019 06:10

Mathematics, 02.09.2019 06:10




Physics, 02.09.2019 06:10