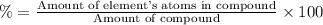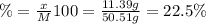Chemistry, 01.07.2019 11:30 cmflores3245
A50.51g sample of a compound made from phosphorus and chlorine is decomposed. analysis of the products showed that 11.39 g of phosphorus atoms were produced. answer using three significant figures. what is the percent by mass of phosphorus? % what is the percent by mass of chlorine? %

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1
You know the right answer?
A50.51g sample of a compound made from phosphorus and chlorine is decomposed. analysis of the produc...
Questions



Chemistry, 08.12.2020 23:50

Mathematics, 08.12.2020 23:50




Mathematics, 08.12.2020 23:50



Mathematics, 08.12.2020 23:50

English, 08.12.2020 23:50


Mathematics, 08.12.2020 23:50


History, 08.12.2020 23:50



Mathematics, 08.12.2020 23:50

Mathematics, 08.12.2020 23:50