Ethane (C2H6) combines with oxygen to
yield carbon dioxide and water.
Let's begin by writing...

Chemistry, 08.12.2020 23:50 McKenzie8409
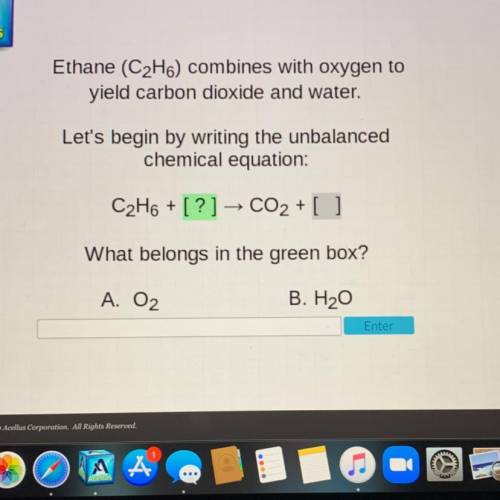
Ethane (C2H6) combines with oxygen to
yield carbon dioxide and water.
Let's begin by writing the unbalanced
chemical equation:

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 05:00
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1
You know the right answer?
Questions

Mathematics, 07.12.2019 18:31


History, 07.12.2019 18:31


Computers and Technology, 07.12.2019 18:31

Social Studies, 07.12.2019 18:31



Health, 07.12.2019 18:31

Social Studies, 07.12.2019 18:31


Mathematics, 07.12.2019 18:31


English, 07.12.2019 18:31



Mathematics, 07.12.2019 18:31





