
Chemistry, 04.07.2019 08:30 bakerj8395
What volume of 0.307 m naoh must be added to 200.0ml of 0.425m acetic acid (ka = 1.75 x 10-5 ) to produce a buffer of ph = 4.250?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
What volume of 0.307 m naoh must be added to 200.0ml of 0.425m acetic acid (ka = 1.75 x 10-5 ) to pr...
Questions


English, 31.05.2020 04:59



Mathematics, 31.05.2020 04:59


Mathematics, 31.05.2020 04:59



Social Studies, 31.05.2020 04:59








Mathematics, 31.05.2020 04:59


Mathematics, 31.05.2020 04:59

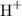 than hydroxide ions
than hydroxide ions 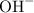 . The equilibrium equation shall thus contain protons rather than a combination of water and hydroxide ions as the reacting species.
. The equilibrium equation shall thus contain protons rather than a combination of water and hydroxide ions as the reacting species.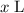 of the 0.307
of the 0.307 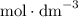 sodium hydroxide solution was added to the acetic acid. Based on previous reasoning,
sodium hydroxide solution was added to the acetic acid. Based on previous reasoning,  is sufficiently small that acetic acid was in excess, and no hydroxide ion has yet been produced in the solution. The solution would thus contain
is sufficiently small that acetic acid was in excess, and no hydroxide ion has yet been produced in the solution. The solution would thus contain 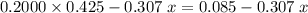 moles of acetic acid and
moles of acetic acid and 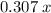 moles of acetate ions.
moles of acetate ions.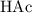 denotes an acetic acid molecule and
denotes an acetic acid molecule and 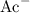 denotes an acetate ion. The RICE table below resembles the hydrolysis equilibrium going on within the buffer solution.
denotes an acetate ion. The RICE table below resembles the hydrolysis equilibrium going on within the buffer solution.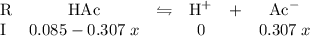
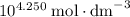 . There were no proton in the buffer solution before the hydrolysis of acetic acid. Therefore the table shall have an increase of
. There were no proton in the buffer solution before the hydrolysis of acetic acid. Therefore the table shall have an increase of 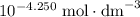 in proton concentration in the third row. Atoms conserve. Thus the concentration increase of protons by
in proton concentration in the third row. Atoms conserve. Thus the concentration increase of protons by 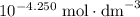 would correspond to a decrease in acetic acid concentration and an increase in acetate ion concentration by the same amount. That is:
would correspond to a decrease in acetic acid concentration and an increase in acetate ion concentration by the same amount. That is: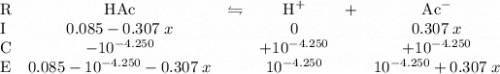
![\text{K}_{a} = [\text{H}^{+}] \cdot [\text{Ac}^{-}] / [\text{HAc}]\\\phantom{\text{K}_{a}} = 10^{-4.250} \times (10^{-4.250} + 0.307 \; x) / (0.085 - 10^{-4.250} - 0.307 \; x)](/tpl/images/0049/5512/989e8.png)
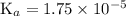
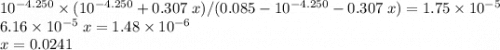
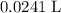 of sodium hydroxide to produce this buffer solution.
of sodium hydroxide to produce this buffer solution.

