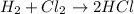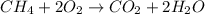Chemistry, 05.07.2019 12:30 izzysmith6836
Identify the type of reaction represented by each equation. a: h2 + cl2 → 2hcl b: ch4 + 2o2 → co2 + 2h2o equation a represents a reaction, and equation b represents reaction. choices for both blanks combustion decombustion synthesis double displacement single displacement choose one for each blank there are some notes and examples in the images my opinions are 1. 2blank--combustion tell me if im correct 16pionts

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 23.06.2019 08:30
If you had to research a particular disease or area of concern in veterinary medicine and science, which one would you choose? why?
Answers: 1
You know the right answer?
Identify the type of reaction represented by each equation. a: h2 + cl2 → 2hcl b: ch4 + 2o2 → co2...
Questions


Mathematics, 13.01.2020 14:31

Arts, 13.01.2020 14:31

History, 13.01.2020 14:31

Mathematics, 13.01.2020 14:31



Mathematics, 13.01.2020 14:31

World Languages, 13.01.2020 14:31


Mathematics, 13.01.2020 14:31

Mathematics, 13.01.2020 14:31





Mathematics, 13.01.2020 14:31

History, 13.01.2020 14:31

Business, 13.01.2020 14:31

Mathematics, 13.01.2020 14:31