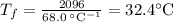Chemistry, 07.07.2019 04:30 IsabellaGracie
What would be the final temperature of the system if 21.2 g of lead at 113 ◦c is dropped into 14.8 g of water at 28.9 ◦c in an insulated container? the specific heat of lead is 0.128 j/g◦c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
What would be the final temperature of the system if 21.2 g of lead at 113 ◦c is dropped into 14.8 g...
Questions



Chemistry, 14.01.2021 02:00

Mathematics, 14.01.2021 02:00

History, 14.01.2021 02:00

Mathematics, 14.01.2021 02:00

Mathematics, 14.01.2021 02:00


Mathematics, 14.01.2021 02:00


German, 14.01.2021 02:00

Mathematics, 14.01.2021 02:00





Mathematics, 14.01.2021 02:00

History, 14.01.2021 02:00


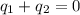
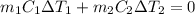
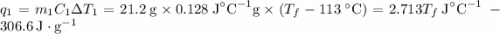
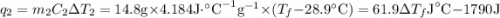
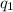 and
and 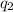 and combinibg like terms, we get
and combinibg like terms, we get![168.0\text{T}_{f}\: ^{\circ}\text{C} ^{-1}\:- 2096 = 0]\\](/tpl/images/0060/4306/42ee8.png)