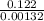Chemistry, 11.07.2019 11:30 taniyahbenyamin2
The decomposition reaction of a to b has a rate constant of 0.00132 s-1: a → 2b if the initial concentration of a is 0.156 m, how long will it take for the concentration of a to decrease by 78.1%

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
The decomposition reaction of a to b has a rate constant of 0.00132 s-1: a → 2b if the initial conc...
Questions


Chemistry, 17.03.2020 20:01


Mathematics, 17.03.2020 20:01

Mathematics, 17.03.2020 20:01




Mathematics, 17.03.2020 20:01



Physics, 17.03.2020 20:02


Biology, 17.03.2020 20:02

Chemistry, 17.03.2020 20:02





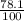 x 0.156
x 0.156