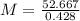The element antimony has an atomic weight of 122 and consists of two stable isotopes antimony-121 and antimony-123. the isotope antimony-121 has a mass of 121 amu and a percent natural abundance of 57.3 %. the isotope antimony-123 has a percent natural abundance of 42.8 %. what is the mass of antimony-123?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
The element antimony has an atomic weight of 122 and consists of two stable isotopes antimony-121 an...
Questions

Mathematics, 13.11.2020 20:10

Mathematics, 13.11.2020 20:10


Biology, 13.11.2020 20:10

History, 13.11.2020 20:10

Mathematics, 13.11.2020 20:10


Advanced Placement (AP), 13.11.2020 20:10


Arts, 13.11.2020 20:10


Mathematics, 13.11.2020 20:10

Chemistry, 13.11.2020 20:10

World Languages, 13.11.2020 20:10

History, 13.11.2020 20:10

Health, 13.11.2020 20:10


SAT, 13.11.2020 20:10

Geography, 13.11.2020 20:10