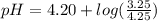Do parts a, b and c a. calculate the ph of 100 ml of an aqueous solution of 0.05 m benzoic acid and 0.025 m benzoate ion. the ka of benzoic acid is 6.3 x 10-5 at 25 ℃. b. calculate the new ph of the solution in part a if you add 10.0 ml of 0.100 m hcl solution. c. calculate the new ph of the solution in part a if you add 15.0 ml of 0.050 m naoh solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Imagine that you own a property that is exactly 2.2 acres large. you want to sell your property, but your realtor tells you that you cannot sell your land by the acre. in order to sell your land you need to determine the area you own in units of square meters? given that there are 1.6 kilometers in 1 mile and 640 acres in 1 square mile, what is the area of land that you own in square meters square meters?
Answers: 2

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

You know the right answer?
Do parts a, b and c a. calculate the ph of 100 ml of an aqueous solution of 0.05 m benzoic acid and...
Questions










Mathematics, 15.04.2020 18:59





Mathematics, 15.04.2020 18:59




Mathematics, 15.04.2020 18:59

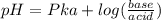
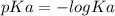
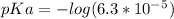
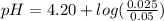
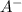 .
.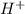 added = 0.100(10.0) = 1
added = 0.100(10.0) = 1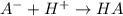
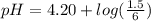
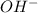 added to the original buffer = 0.05(15.0) = 0.75
added to the original buffer = 0.05(15.0) = 0.75