
Chemistry, 17.07.2019 20:00 kyllow5644
Acompound has a percent composition of 40.0% carbon, 6.72% hydrogen and 53.28% oxygen. if its molar mass is 180 g/mol, what is its molecular formula?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

You know the right answer?
Acompound has a percent composition of 40.0% carbon, 6.72% hydrogen and 53.28% oxygen. if its molar...
Questions

English, 01.10.2019 18:30

Computers and Technology, 01.10.2019 18:30

Computers and Technology, 01.10.2019 18:30


Mathematics, 01.10.2019 18:30















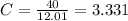
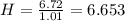
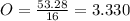
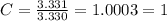
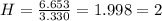
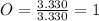
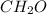
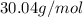
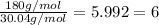
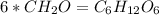
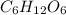 .
.

