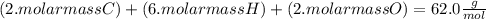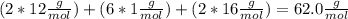Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2
You know the right answer?
An organic compound is composed of 38.7% c, 9.70% h, 51.6% o. the compound has a molecular formula m...
Questions

Mathematics, 30.11.2021 21:10


Chemistry, 30.11.2021 21:10

Business, 30.11.2021 21:10



Mathematics, 30.11.2021 21:10




Mathematics, 30.11.2021 21:10








Computers and Technology, 30.11.2021 21:10

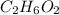
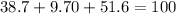
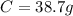
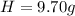
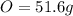
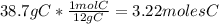
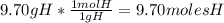
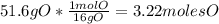
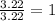
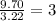
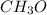 , but you should find the molecular formula taking in account the molar mass of the compound, so:
, but you should find the molecular formula taking in account the molar mass of the compound, so: