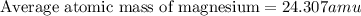Chemistry, 19.07.2019 19:00 erikagibson3414
The naturally occurring isotopes of magnesium are magnesium-24. magnesium-25, and magnesium-26. magnesium-24 has an abundance of 78.994% and a mass of 23.985 amu. magnesium-25 has an abundance of 10.001% and a mass of 24.986 amu. magnesium-26 has an abundance of 11.013% and a mass of 25.983 amu. calculate the average atomic mass of magnesium.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1

Chemistry, 23.06.2019 08:00
Which of the following notations would be the appropriate and final way to display the formula for magnesium chloride a. mgcl2 b. mg+2cl–1 c. mgcl2 d. mgcl
Answers: 2
You know the right answer?
The naturally occurring isotopes of magnesium are magnesium-24. magnesium-25, and magnesium-26. magn...
Questions

Social Studies, 10.07.2019 20:20

Mathematics, 10.07.2019 20:20







Mathematics, 10.07.2019 20:20

Mathematics, 10.07.2019 20:20





Biology, 10.07.2019 20:20


Mathematics, 10.07.2019 20:20

Mathematics, 10.07.2019 20:20

Mathematics, 10.07.2019 20:20

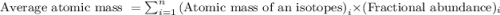 .....(1)
.....(1)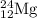 isotope:
isotope: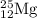 isotope:
isotope: isotope:
isotope:![\text{Average atomic mass of magnesium}=[(23.985\times 0.78994)+(24.986\times 0.10001)+(25.983\times 0.11013)]](/tpl/images/0108/8899/cfd57.png)