
Chemistry, 24.09.2019 17:40 annamcda301
Check all correct statements concerning pentyne.
contains 3 carbons
contains 5 carbons
contains 7 carbons
has only single bonds
has a double bond
has a triple bond
is an alkyne
is an alkene
is an alkane
which statement is not true of alkenes?
they are unsaturated.
they are reactive.
they have double bonds.
they are more stable than alkanes.
butene would have 4 carbon atoms and a bond.
single
triple
double
which type of reaction to alkenes generally undergo?
substitution
displacement
redox
addition
if the carbon atom of hydrocarbon is bonded to less than 4 other atoms, the hydrocarbon is considered
aromatic
diluted
saturated
unsaturated
which of the following apply to unsaturated hydrocarbons? select all that apply.
they do not have isomers.
they are more stable than saturated hydrocarbons.
they will react readily with other elements and compounds.
they have double and triple bonds.
they only have single bonds.
a straight chain hydrocarbon with the formula c5h10:
has a triple c-c bond
has a double c-c bond
is essentially inert
is unstable and extremely reactive
what is the general formula for alkenes?
cnh2n+2
cnh2n
cnh2n+1
ch2n+2
choose the statements about benzene that are correct.
all benzene hydrocarbons are cnhn+2
all c-c bonds share 2 pairs of electrons.
three c-c bonds share 2 pairs of electrons.
all of the c-c bonds are actually identical.
the structure of the molecule is closed like a ring structure.
the last one is attached as a pic.
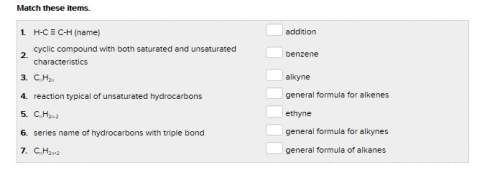

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1
You know the right answer?
Check all correct statements concerning pentyne.
contains 3 carbons
contains 5 carbons<...
contains 3 carbons
contains 5 carbons<...
Questions




Computers and Technology, 15.04.2020 02:16



Mathematics, 15.04.2020 02:16

Computers and Technology, 15.04.2020 02:16




Spanish, 15.04.2020 02:16


Physics, 15.04.2020 02:16



Mathematics, 15.04.2020 02:16

Chemistry, 15.04.2020 02:16

Mathematics, 15.04.2020 02:16



