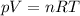Physics, 22.07.2019 14:00 lilswetheart2007
This equation is known as the ideal gas law, and it can be used to predict the behavior of many gases at relatively low pressure. from this equation, you can see that as the temperature of a gas increases, a) the pressure of the gas increases. b) the number of moles of gas will go down. c) either the pressure of the gas, the volume of the gas, or both, will increase. d) either the pressure of the gas, the volume of the gas, or both, will decrease.

Answers: 1


Another question on Physics

Physics, 22.06.2019 05:00
At time t=0, a particle is located at the point (3,6,9). it travels in a straight line to the point (5,2,7), has speed 8 at (3,6,9) and constant acceleration 2i−4j−2k. find an equation for the position vector of the particle.
Answers: 2

Physics, 22.06.2019 14:00
How to tell if two objects are in thermal equilibrium 1.the objects have the same temperature 2.the objects are the same size 3.the objects are touching each other 4.the objects temperatures are changing
Answers: 1

Physics, 22.06.2019 14:40
According to valence bond theory, which orbitals overlap in the formation of the bond in hf according to valence bond theory, which orbitals overlap in the formation of the bond in hf 2s on h and 2p on f 1s on h and 2s on f 1s on h and 1p on f 1s on h and 2p on f 1s on h and 3p on f
Answers: 3

Physics, 22.06.2019 18:30
Against his financial advisor's advice, frank has decided to invest his money in some risky stocks because he once made quite a bit of money in the stock market. his decision illustrates a. the representativeness heuristic b. overconfidence c. the availability heuristic d. confirmation bias select the best answer from the choices provided
Answers: 3
You know the right answer?
This equation is known as the ideal gas law, and it can be used to predict the behavior of many gase...
Questions

Arts, 08.12.2020 01:00

Social Studies, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00



Mathematics, 08.12.2020 01:00


Mathematics, 08.12.2020 01:00

Computers and Technology, 08.12.2020 01:00






Social Studies, 08.12.2020 01:00