
Physics, 28.07.2019 15:30 battlemarshmell
How does the law of conservation of mass apply to this reaction: c2h4 + o2 → h2o + co2? a. the equation needs to be balanced. there are fewer oxygen atoms in the equation than hydrogen or carbon. b. only the oxygen needs to be balanced. there are equal numbers of hydrogen and carbon. c. the law of conservation of mass has already been applied. there is an equal number of each element on both sides of the equation. d. each element needs to be balanced.

Answers: 2


Another question on Physics

Physics, 21.06.2019 22:40
Explain vector addition, triangle method and parallelogram method
Answers: 1

Physics, 22.06.2019 02:20
According to newton’s first law of motion, which force is expected to cause a body to accelerate?
Answers: 1

Physics, 22.06.2019 07:00
Examine the equation. 23490th→23088ra+42he what kind of barrier would you need to block the radioactive particles from this reaction? a.a piece of paper b.a sheet of aluminum foil c. a two-inch block of lead d. a solid concrete block
Answers: 1

Physics, 22.06.2019 08:50
You are a sales representative for a company that makes a new alternate fuel for vehicles. you have prepared a presentation for the environmental engineers to sell your new product. what question do you expect the audience to ask regarding whether the new fuel will cause less damage to the environment? a. do we have to change any parts of the vehicle to use this alternate fuel? b. will the vehicles get better fuel mileage with the alternate fuel? c. how much greenhouse gas does your fuel produce compared with current fuel sources? d. is the alternate fuel more expensive than fossil fuel?
Answers: 2
You know the right answer?
How does the law of conservation of mass apply to this reaction: c2h4 + o2 → h2o + co2? a. the eq...
Questions

Computers and Technology, 25.08.2019 07:50

English, 25.08.2019 07:50


Biology, 25.08.2019 07:50

Social Studies, 25.08.2019 07:50


History, 25.08.2019 07:50



English, 25.08.2019 07:50

Mathematics, 25.08.2019 07:50

Mathematics, 25.08.2019 07:50

Mathematics, 25.08.2019 07:50


Mathematics, 25.08.2019 07:50


English, 25.08.2019 07:50

Mathematics, 25.08.2019 07:50

Chemistry, 25.08.2019 07:50

Mathematics, 25.08.2019 07:50

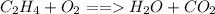 , the are 2 carbon atoms on reactant side as opposed to 1 carbon atom on product side, there are 4 hydrogen atoms on reactant side as opposed to 2 hydrogen atoms on product side, and there are 2 oxygen atoms on reactant side as opposed to 3 atoms of oxygen on product side. To balance the equation we add a coefficient of 3 on O_2 and on the product side we put a coefficient of 2 on both water
, the are 2 carbon atoms on reactant side as opposed to 1 carbon atom on product side, there are 4 hydrogen atoms on reactant side as opposed to 2 hydrogen atoms on product side, and there are 2 oxygen atoms on reactant side as opposed to 3 atoms of oxygen on product side. To balance the equation we add a coefficient of 3 on O_2 and on the product side we put a coefficient of 2 on both water 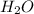 and carbon dioxide
and carbon dioxide 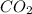 .
.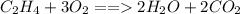 .
.

