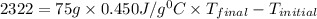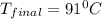Physics, 03.08.2019 16:00 AlaskaAirlines
Suppose that 0.95 g of water condenses on a 75.0 g block of iron that is initially at 22 â°c. if the heat released during condensation is used only to warm the iron block, what is the final temperature (in â°c) of the iron block? (assume a constant enthalpy of vaporization for water of 44.0 kj/mol.)

Answers: 2


Another question on Physics

Physics, 21.06.2019 19:30
Molten iron fills a mould, which has a volume of 200 cm cubed. calculate the volume when the iron cools and solidifies.
Answers: 1

Physics, 22.06.2019 03:30
As part of an industrial process, air as an ideal gas at 10 bar, 400k expands at steady state through a valve to a pressure of 4 bar. the mass flow rate of air is 0.5 kg/s. the air then passes through a heat exchanger where it is cooled to a temperature of 295k with negligible change in pressure. the valve can be modeled as a throttling process, and kinetic and potential energy effects can be neglected. (a) for a control volume enclosing the valve and heat exchanger and enough of the local surroundings that the heat transfer occurs at the ambient temperature of 295 k, determine the rate of entropy production, in kw/k. (b) if the expansion valve were replaced by an adiabatic turbine operating isentropically, what would be the entropy production? compare the results of parts (a) and (b) and discuss.
Answers: 3

Physics, 22.06.2019 07:00
Oxygen and hydrogen gas are at the same temperature t.what is the ratio of kinetic energies of oxygen molecule and hydrogen molecule if oxygen is 16 times heavier than hydrogen.
Answers: 3

Physics, 22.06.2019 11:30
While you are driving in the lane next to the curb on a multi-lane road the car on your left suddenly moves toward you lane. they are about toy crash into your front fender. you
Answers: 2
You know the right answer?
Suppose that 0.95 g of water condenses on a 75.0 g block of iron that is initially at 22 â°c. if the...
Questions


English, 13.05.2021 09:00

Mathematics, 13.05.2021 09:00






English, 13.05.2021 09:00



English, 13.05.2021 09:00

Mathematics, 13.05.2021 09:00


Mathematics, 13.05.2021 09:00

Mathematics, 13.05.2021 09:00

Computers and Technology, 13.05.2021 09:00

Spanish, 13.05.2021 09:00

English, 13.05.2021 09:00

Mathematics, 13.05.2021 09:00


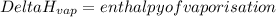

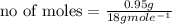


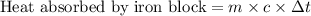
 =change in temperature
=change in temperature