
Physics, 02.09.2019 10:30 mamas12345
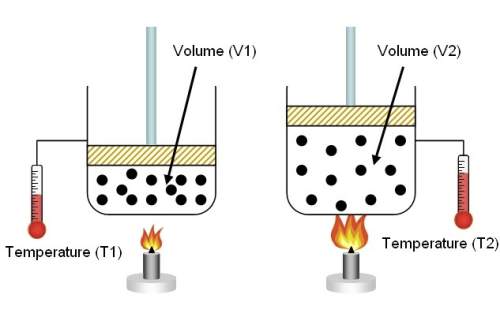
In the model above, a volume of gas is held in a container. over time, the container is heated by the flame at the bottom. all but one statement might describe what is happening inside the containers from left (v1) to right (v2).
a) an increase in particle energy causes an increase in pressure.
b) the change in temperature causes the particles to increase in number. c) as the temperature increases, the kinetic energy of the gas also increases. d) an decrease in kinetic energy and pressure causes an increase in the volume of the container.

Answers: 2


Another question on Physics

Physics, 21.06.2019 19:00
Find the momentum for a 2.6-kg brick parachuting straight downward at a constant speed of 8.4 m/s .
Answers: 3

Physics, 22.06.2019 04:30
Asystem containing an ideal gas at a constant pressure of 1.22×10^5 pa gains 2140 j of heat. during the process, the internal energy of the system increases by 2320 j. what is the change in volume of the gas?
Answers: 3

Physics, 22.06.2019 06:00
When you push downward on a book at rest on a table, you feel an upward force. does this force depend on friction? defend your answer.
Answers: 2

You know the right answer?
In the model above, a volume of gas is held in a container. over time, the container is heated by th...
Questions

Physics, 10.07.2019 09:30



Biology, 10.07.2019 09:30

Chemistry, 10.07.2019 09:30


Geography, 10.07.2019 09:30

History, 10.07.2019 09:30

Mathematics, 10.07.2019 09:30

Mathematics, 10.07.2019 09:30

Mathematics, 10.07.2019 09:30

Mathematics, 10.07.2019 09:30



English, 10.07.2019 09:30

Mathematics, 10.07.2019 09:30






