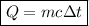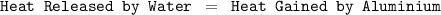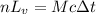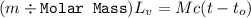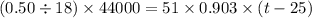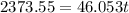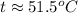Suppose that 0.50 g of water at 25 ∘c condenses on the surface of a 51-g block of aluminum that is initially at 25 ∘c. if the heat released during condensation goes only toward heating the metal, what is the final temperature (in ∘c) of the metal block? (the specific heat capacity of aluminum is 0.903 j/g∘c and the heat of vaporization of water at 25 ∘c is 44.0 kj/mol.)

Answers: 1


Another question on Physics


Physics, 22.06.2019 05:00
Modern physics a photon emitted from an excited hydrogen atom has an energy of 3.02 electron volts. which electron energy-level transition would produce this photon? a. n=1 to n=6 b. n=2 to n=6 c. n=6 to n=1 d. n=6 to n=2 i chose b but the correct answer is d can someone tell me why? and what's the difference?
Answers: 1

Physics, 22.06.2019 07:30
Choose all the answers that apply. our solar system: is in a spiral galaxy no longer includes pluto is made mostly of empty space is in the andromeda galaxy is the only known system that supports life
Answers: 3

Physics, 22.06.2019 09:00
A100 kg running back runs at 5m/s into a stationary linebacker. it takes 0.5 for the running back to be completely stopped
Answers: 3
You know the right answer?
Suppose that 0.50 g of water at 25 ∘c condenses on the surface of a 51-g block of aluminum that is i...
Questions




Mathematics, 30.11.2020 02:00

Mathematics, 30.11.2020 02:00

Physics, 30.11.2020 02:00


Biology, 30.11.2020 02:00

Mathematics, 30.11.2020 02:00


Mathematics, 30.11.2020 02:00

Mathematics, 30.11.2020 02:00


Mathematics, 30.11.2020 02:00


Mathematics, 30.11.2020 02:00


Physics, 30.11.2020 02:00