
Physics, 07.12.2021 20:00 emilyy4757
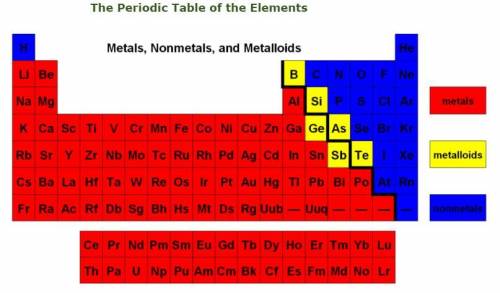
By looking at the relative positions of the elements calcium, Ca, fluorine, F, sulfur, S, and oxygen, O, in the Periodic Table, which TWO descriptions are TRUE about calcium fluoride, CaF2, and sulfur dioxide, SO2?
CaF2 is an ionic compound, and SO2i is a covalent compound.
CaF2 is formed by covalent bonds, and SO2 is formed by ionic bonds.
CaF2 cannot conduct electricity when dissolved in water, but SO2 can.
CaF2 is a gas at room temperature, while SO2 has a high boiling point.
CaF2 has a high boiling point, while SO2 is a gas at room temperature.
CaF2 is soft at room temperature, while SO2 is hard and forms crystals.

Answers: 1


Another question on Physics

Physics, 22.06.2019 11:00
Engineers find a new metal that is stronger than steel but much lighter. this material is also significantly cheaper than what is currently used for most aircraft, available in large quantities, and easy to manufacture. the engineers are excited because this new material will lower the costs of buying and operating airplanes for companies. what is probably the best step for the engineers to make next?
Answers: 2

Physics, 22.06.2019 12:00
Explain why electric current cannot exist if a current doesn't have a voltage source.
Answers: 2

Physics, 22.06.2019 16:30
Humidity is to blame for that muggy, steamy feeling you experience on some hot summer days. what gas in the atmosphere causes humidity? a) oxygen b) hydrogen c) nitrogen d) water vapor
Answers: 1

Physics, 23.06.2019 04:31
Atruck travels at an average speed of 70 km/hr. if it travels 100 km in the first hour, how far will it go the second hour of a 2 hour trip? if you could also give me the steps that would great.
Answers: 3
You know the right answer?
By looking at the relative positions of the elements calcium, Ca, fluorine, F, sulfur, S, and oxygen...
Questions

Mathematics, 18.01.2020 19:31


Mathematics, 18.01.2020 19:31

English, 18.01.2020 19:31



Physics, 18.01.2020 19:31


English, 18.01.2020 19:31

Mathematics, 18.01.2020 19:31

Mathematics, 18.01.2020 19:31

History, 18.01.2020 19:31


Mathematics, 18.01.2020 19:31


Health, 18.01.2020 19:31

Biology, 18.01.2020 19:31

History, 18.01.2020 19:31




