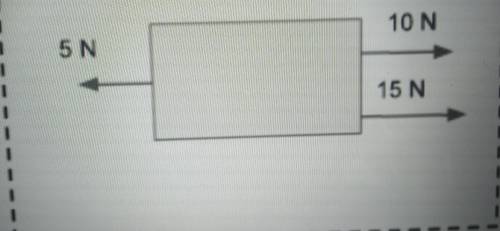
For these last four question list the net force and direction.
...

Answers: 2


Another question on Physics

Physics, 22.06.2019 05:50
Acylinder with a movable piston contains 11.7 moles of a monatomic ideal gas at a pressure of 1.32×10^5 pa. the gas is initially at a temperature of 300 k. an electric heater adds 43200 j of energy into the gas while the piston moves in such a way that the pressure remains constant. cp=20.79 j k^−1 mol^−1 for a monatomic ideal gas, and that the number of gas molecules is equal to avogadro's number (6.022×10^23) times the number of moles of the gas. (a) what is the temperature of the gas after the energy is added? (b) what is the change in volume of the gas? (c) how much work is done by the gas during this process?
Answers: 3

Physics, 22.06.2019 06:30
At very high pressures, gases become and will eventually a) more dense; become hotter b) more dense; change to a liquid or solid c) less dense; combust d) less dense; turn into a liquid
Answers: 1

Physics, 22.06.2019 15:30
What is a view of science and psychology that says the value of knowledge depends on its usefulness? a. pragmatism b. psychotherapy c. physiology
Answers: 2

Physics, 22.06.2019 20:30
You are doing a science experiment with a fahrenheit thermometer. your data must be in degrees celsius. if you measure a temperature of 125°f, what is this temperature in degrees celsius
Answers: 1
You know the right answer?
Questions


Social Studies, 05.05.2020 14:48

Mathematics, 05.05.2020 14:48

Mathematics, 05.05.2020 14:48

Mathematics, 05.05.2020 14:48

Mathematics, 05.05.2020 14:48

Physics, 05.05.2020 14:48




Mathematics, 05.05.2020 14:48

Mathematics, 05.05.2020 14:48




Chemistry, 05.05.2020 14:48

Biology, 05.05.2020 14:48

Computers and Technology, 05.05.2020 14:48

Biology, 05.05.2020 14:48

Mathematics, 05.05.2020 14:48