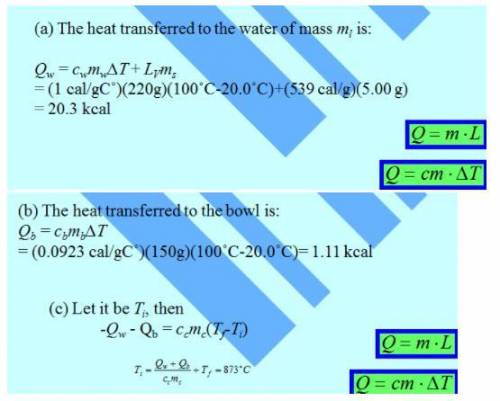
A 150g copper bowl contains 220g of water, both at 20.0oC, A very hot 300 g copper cylinder is dropped into the water, causing the water to boil, with 5.00 g being converted to steam. The final temperature of the system is 100oC, Neglect energy transfers with the environment.
a) How much energy (in calories) is transfered to the water as heat?
b) How much to the bowl?
c) What is the original temperature of the cylinder?

Answers: 1


Another question on Physics

Physics, 22.06.2019 06:00
Which of the following changes will result in a stronger electromagnet? a. using fewer coils of wire b. using a higher voltage c. using a shorter nail d. using a longer wire
Answers: 1

Physics, 22.06.2019 09:00
When a light bulb shines, it gives off light energy and energy. a. heat b. potential c. chemical d. electrical
Answers: 2

Physics, 22.06.2019 12:00
Explain why electric current cannot exist if a current doesn't have a voltage source.
Answers: 2

Physics, 22.06.2019 12:10
An ionic compound can conduct electricity when a it is dissolved in water b its crystals are ground into powder c it is in solid form d it is heated to just below its melting point
Answers: 1
You know the right answer?
A 150g copper bowl contains 220g of water, both at 20.0oC, A very hot 300 g copper cylinder is dropp...
Questions



Mathematics, 12.11.2020 18:30

Biology, 12.11.2020 18:30

Mathematics, 12.11.2020 18:30



Computers and Technology, 12.11.2020 18:30



Mathematics, 12.11.2020 18:30

Mathematics, 12.11.2020 18:30


Biology, 12.11.2020 18:30

Chemistry, 12.11.2020 18:30

Biology, 12.11.2020 18:30

Mathematics, 12.11.2020 18:30


Mathematics, 12.11.2020 18:30

English, 12.11.2020 18:30