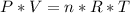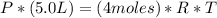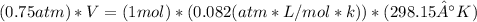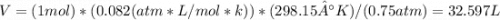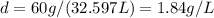Physics, 30.07.2021 05:50 efraruiz02
Un globo contiene 4 moles de un gas ideal con un volúmen de 5,0 L. Si se agregan 8 moles adicionales del gas a presión y temperatura constantes, ¿Cuál será el volumen final del globo? - ¿Cuál es la densidad (en g / L) de un gas con una masa molar de 60 g / mol a 0,75 atm y 27 ° C?

Answers: 3


Another question on Physics

Physics, 20.06.2019 18:04
Abeam has a square cross-section, a x a, where a is the linear dimension, and is subject to a pure bending moment, m. m is known with an uncertainty of 10% and a is known with an uncertainty of 5%. the strength of the material is known to an uncertainty of 12%. find the minimum design factor such that the beam is guaranteed not to fail.
Answers: 3

Physics, 22.06.2019 02:40
What happens when chlorine reacts with bromine? a. electrons move from the chlorine atoms to the bromine atoms. b. electrons move from the bromine atoms to the chlorine atoms. c. electrons are shared between the chlorine atoms and the bromine atoms. d. electrons become delocalized among the atoms.
Answers: 2

Physics, 22.06.2019 14:00
Amass attached to a spring is displaced from its equilibrium position by 5cm and released. the system then oscillates in simple harmonic motion with a period of 1s. if that same mass–spring system is displaced from equilibrium by 10cm instead, what will its period be in this case? a mass attached to a spring is displaced from its equilibrium position by and released. the system then oscillates in simple harmonic motion with a period of . if that same mass–spring system is displaced from equilibrium by instead, what will its period be in this case? a) 0.5sb) 2sc) 1sd) 1.4s
Answers: 2

Physics, 22.06.2019 16:30
Is there a point between a 10 nc charge and a 20 nc charge at which the electric field is zero?
Answers: 2
You know the right answer?
Un globo contiene 4 moles de un gas ideal con un volúmen de 5,0 L. Si se agregan 8 moles adicionales...
Questions









Mathematics, 15.07.2020 01:01

Computers and Technology, 15.07.2020 01:01

Mathematics, 15.07.2020 01:01





Physics, 15.07.2020 01:01