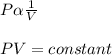What does Boyle's Law state about the relationship between the pressure and volume of an ideal gas at constant temperature?
a) The product of pressure and volume increases as pressure decreases.
b) The sum of pressure and volume is constant.
c) The sum of product and volume decreases as volume increases.
d) The product of pressure and volume is constant.

Answers: 1


Another question on Physics

Physics, 22.06.2019 04:10
Time remainin52: 42the chart shows data for a moving object.which conclusion is best supported by the information inthe chart? time (s)velocity (m/s
Answers: 3

Physics, 22.06.2019 07:40
Which best describes how fluids change as they travel through different portions of the convection currents? they change to solids at the outer portion of the convection currents. they change to solids at the inner portion of the convection currents. they become more dense at the outer portion of the convection currents. they become more dense at the inner portion of the convection currents
Answers: 2

Physics, 22.06.2019 10:20
Assume that a person skiing high in the mountains at an altitude of h = 15100 ft takes in the same volume of air with each breath as she does while walking at sea level. determine the ratio of the mass of oxygen inhaled for each breath at this high altitude compared to that at sea level. assume that the air composition (i.e. % of air that is oxygen) is the same at sea level as it is at 15100 ft.
Answers: 2

Physics, 22.06.2019 13:30
Which is not an example of how an object gains elastic potential energy by stretching? a. jumping on a pogo stick b. pulling on a rubber band c. jumping on a trampoline
Answers: 1
You know the right answer?
What does Boyle's Law state about the relationship between the pressure and volume of an ideal gas a...
Questions



Biology, 22.05.2020 19:57



Mathematics, 22.05.2020 19:57



Mathematics, 22.05.2020 19:58




Social Studies, 22.05.2020 19:58


Mathematics, 22.05.2020 19:58

Mathematics, 22.05.2020 19:58


Mathematics, 22.05.2020 19:58


English, 22.05.2020 19:58