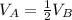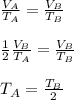Container A and container B hold samples of the same ideal gas. The volume and the pressure of container A is equal to the volume and pressure of container B, respectively. If Container A has half as many molecules of the ideal gas in it as Container B does, then which of the following mathematical statements is correct regarding the absolute temperatures TA and TB in Container A and Container B. respectively?
A. TA = TB/2.
B. TA = 4TB.
C. TA = TB/4.
D. TA = 2TB.
E. TA = TB

Answers: 1


Another question on Physics

Physics, 22.06.2019 07:40
Which lists the fundamental forces in order, from strongest to weakest? strong nuclear, weak nuclear, electromagnetic, gravitational strong nuclear, electromagnetic, weak nuclear, gravitational gravitational, weak nuclear, electromagnetic, strong nuclear electromagnetic, gravitational, strong nuclear, weak nuclear
Answers: 2


Physics, 22.06.2019 12:00
Selma made a diagram to compare convection and radiation. which label belongs in the area marked x? must involve temperature differences between substances or objects only occurs when molecules are in direct contact involves the movement of fluids based on density differences can occur where there is little or no matter
Answers: 1

Physics, 22.06.2019 13:10
Which additional product balances the reaction h2so4 + 2naoh → na2so4 + 2h2o 2oh h2o2 h3o
Answers: 1
You know the right answer?
Container A and container B hold samples of the same ideal gas. The volume and the pressure of conta...
Questions


Chemistry, 16.07.2019 16:30

Geography, 16.07.2019 16:30



Business, 16.07.2019 16:30







English, 16.07.2019 16:30


World Languages, 16.07.2019 16:30