
Two identical cylinders with a movable piston contain 0.7 mol of helium gas at a temperature of 300 K. The temperature of the gas in the first cylinder is increased to 412 K at constant volume by doing work W1 and transferring energy Q1 by heat. The temperature of the gas in the second cylinder is increased to 412 K at constant pressure by doing work W2 while transferring energy Q2 by heat.
Required:
Find ÎEint, 1, Q1, and W1 for the process at constant volume.

Answers: 2


Another question on Physics

Physics, 21.06.2019 22:00
To run the physics cart, the fan speed of the cart is manipulated. this is the (blank) variable. the cart accelerates due to the speed of the fan. acceleration is therefore the (blank) variable. a “constant” is a parameter that stays the same regardless of the variables. one parameter of the cart that is held constant is the (blank) .
Answers: 1

Physics, 22.06.2019 05:30
Física: un futbolista patea hacia el arco con una velocidad de 15m/ah, calcular: a)el alcance para un ángulo de tiro de 25? , b) el tiempo que el balón permanece en el aire.
Answers: 3

Physics, 22.06.2019 05:30
Imagine that someone pushes one marble toward a motionless marble. would there still be action-reaction forces involved in the collision? how might the marbles’ motions be changed? ?
Answers: 1

Physics, 22.06.2019 06:30
=force × distance a. work b. velocity c. pressure d. momentum
Answers: 1
You know the right answer?
Two identical cylinders with a movable piston contain 0.7 mol of helium gas at a temperature of 300...
Questions

Mathematics, 04.02.2021 23:10

Geography, 04.02.2021 23:10

Mathematics, 04.02.2021 23:10


Mathematics, 04.02.2021 23:10

World Languages, 04.02.2021 23:10


Mathematics, 04.02.2021 23:10

Mathematics, 04.02.2021 23:10

Mathematics, 04.02.2021 23:10

Mathematics, 04.02.2021 23:10

English, 04.02.2021 23:10

Mathematics, 04.02.2021 23:10

Spanish, 04.02.2021 23:10

Mathematics, 04.02.2021 23:10


Mathematics, 04.02.2021 23:10

Chemistry, 04.02.2021 23:10

Chemistry, 04.02.2021 23:10

Social Studies, 04.02.2021 23:10

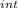 ,₁ = 977.7 J , Q₁ = 977.7 J and W₁ = 0 J
,₁ = 977.7 J , Q₁ = 977.7 J and W₁ = 0 J  = 300 K, T
= 300 K, T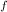 = 412 K, n = 0.7 mol
= 412 K, n = 0.7 mol 

