
Physics, 08.06.2021 01:00 gmoney1973
The liquid and gaseous state of hydrogen are in thermal equilibrium at 20.3 K. Even though it is on the point of condensation, model the gas as ideal and determine the most probable speed of the molecules (in m/s). What If? At what temperature (in K) would an atom of xenon in a canister of xenon gas have the same most probable speed as the hydrogen in thermal equilibrium at 20.3 K?

Answers: 1


Another question on Physics

Physics, 21.06.2019 15:00
How much work would be needed to raise the payload from the surface of the moon (i.e., x = r) to an altitude of 5r miles above the surface of the moon (i.e., x = 6r)?
Answers: 2

Physics, 22.06.2019 04:00
Ametal ball with a mass of 0.028 kg is dropped from rest at a height of 1.0 meters above the ground. assuming the energy in the ball is conserved, how much kinetic energy will the ball have when it is 0.5 meters above the ground?
Answers: 1

Physics, 22.06.2019 07:30
The slope of a velocity time graph over any interval of time gives the during that interval
Answers: 2

Physics, 22.06.2019 10:30
The freezing and boiling point of a substance changes as the air pressure around it changes. for example, at a lower air pressure (higher altitude) it is easier for water molecules to escape from liquid into the air. in a high altitude city such as denver, colorado compared to a sea-level city such as houston, texas, water
Answers: 2
You know the right answer?
The liquid and gaseous state of hydrogen are in thermal equilibrium at 20.3 K. Even though it is on...
Questions



English, 20.10.2021 14:00







Arts, 20.10.2021 14:00


SAT, 20.10.2021 14:00


French, 20.10.2021 14:00


SAT, 20.10.2021 14:00


Mathematics, 20.10.2021 14:00

History, 20.10.2021 14:00

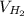 = √( 2RT /
= √( 2RT / 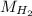 )
)

