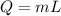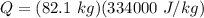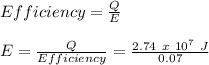Physics, 20.05.2021 19:20 lilybear1700
An ice-making machine inside a refrigerator operates in a Carnot cycle. It takes heat from liquid water at 0.0 degrees Celsius and rejects heat to a room at a temperature of 20.6 degrees Celsius. Suppose that liquid water with a mass of 82.1 kg at 0.0 degrees Celsius is converted to ice at the same temperature. Take the heat of fusion for water to be Lf=3.34×105 J/kg.
A. How much heat |QH| is rejected to the room?
B. How much energy E must be supplied to the device?

Answers: 1


Another question on Physics

Physics, 22.06.2019 15:30
What are the similarities & differences between a thermistor and a light dependent resistor in physics?
Answers: 1


Physics, 23.06.2019 00:30
Which of the following conditions lead you to see an absorption line spectrum from a cloud of gas in interstellar space? the cloud is extremely hot. the cloud is visible primarily because it reflects light from nearby stars. the cloud is cool and lies between you and a hot star. the cloud is cool and very dense, so that you cannot see any objects that lie behind it.
Answers: 3

Physics, 23.06.2019 02:00
Calculate the numbers of photons having a wavelength of 10.0 mm required to produce 1.0 kj of energycalculate the numbers of photons having a wavelength of 10.0 mm required to produce 1.0 kj of energy
Answers: 1
You know the right answer?
An ice-making machine inside a refrigerator operates in a Carnot cycle. It takes heat from liquid wa...
Questions


English, 07.07.2020 20:01

Mathematics, 07.07.2020 20:01

Biology, 07.07.2020 20:01

Mathematics, 07.07.2020 20:01




History, 07.07.2020 20:01

History, 07.07.2020 20:01

Physics, 07.07.2020 20:01





Mathematics, 07.07.2020 20:01


English, 07.07.2020 20:01


Biology, 07.07.2020 20:01