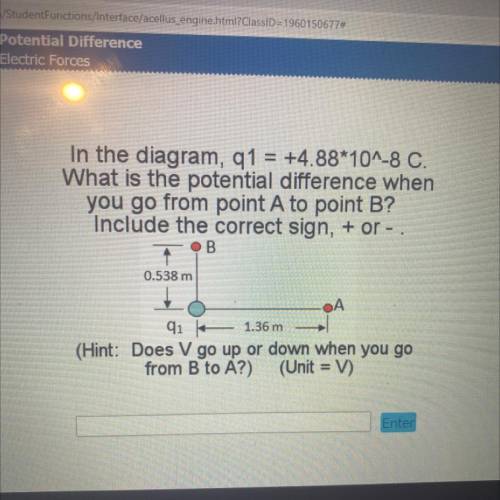
In the diagram, q1 = +4.88*10^-8 C.
What is the potential difference when
you go from point A...


Answers: 2


Another question on Physics

Physics, 21.06.2019 19:30
The lights used by mark watley (played by matt damon) during the film the martian seem to be metal halide lamps. metal halide lamps are filled with vaporized mercury and metal-halogen compounds. when an electric current is passed through the lamp, the tube begins to glow a bright white/blue color. if you were to pass this light through a prism to separate the individual light frequencies, you would see a rainbow just as you would if using natural sunlight because of the complexity of the metal halide gas and the vast amount of possible electron transitions. (the study of light in this way is known as spectroscopy and allows astronomers to know exactly what atoms compose distant stars, simply by looking at the light they emit. the spectral lines an atom produces uniquely identifies that atom just like a fingerprint uniquely identifies a person. the momentum equation and energy equation that we have used above can be combined to give the following equation: c = e p where again p is the phonon momentum, e is the photon energy and c is the speed of light. when you divide the photon energy found in #6 by the photon momentum found in #4, do you get the speed of light? (if not, check your work for questions #4 through #6). yes no
Answers: 2

Physics, 22.06.2019 06:10
Which transition by an electron will release the greatest amount of energy? oa ob oc od
Answers: 2

Physics, 22.06.2019 07:30
Some material consisting of a collection of microscopic objects is kept at a high temperature. a photon detector capable of detecting photon energies from infrared through ultraviolet observes photons emitted with energies of 0.3 ev, 0.5 ev, 0.8 ev, 2.0ev, 2.5ev, and 2.8ev. these are the only photon energies observed. (a) draw and label a possible energy-level diagram for one of the microscopic objects, which has four bound states. on the diagram, indicate the transitions corresponding to the emitted photons. explain briefly. (b) would a spring–mass model be a good model for these microscopic objects? why or why not? (c) the material is now cooled down to a very low temperature, and the photon detector stops detecting photon emissions. next, a beam of light with a continuous range of energies from infrared through ultraviolet shines on the material, and the photon detector observes the beam of light after it passes through the material. what photon energies in this beam of light are observed to be significantly reduced in intensity (“dark absorption lines”)? explain briefly.
Answers: 3

Physics, 22.06.2019 09:00
When a light bulb shines, it gives off light energy and energy. a. heat b. potential c. chemical d. electrical
Answers: 2
You know the right answer?
Questions


Mathematics, 08.12.2019 23:31

Physics, 08.12.2019 23:31

Mathematics, 08.12.2019 23:31




Physics, 08.12.2019 23:31








Mathematics, 08.12.2019 23:31

Social Studies, 08.12.2019 23:31

Mathematics, 08.12.2019 23:31

Health, 08.12.2019 23:31