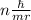Physics, 12.05.2021 01:00 payshencec21
The key insight that Bohr introduced to his model of the atom was that the angular momentum of the electron orbiting the nucleus was quantized. He introduced the postulate that the angular momentum could only come in quantities of nh/(2π), where h is Planck's constant and n is a nonnegative integer (0,1,2,3,…). Given this postulate, what are the allowable values for the velocity v of the electron in the Bohr atom? Recall that, in circular motion, angular momentum is given by the formula L= mvr.

Answers: 3


Another question on Physics

Physics, 22.06.2019 05:00
The image below shows numerous volcanic mountains in the pacific northwest. what is the most likely cause of the volcanic and earthquake activity in this region?
Answers: 2

Physics, 22.06.2019 08:00
What is the average speed of a car that travels 40 mph for 1 hour and 60 mph in another hour?
Answers: 1

Physics, 22.06.2019 18:50
An insulated thermos contains 148 g of water at 72.7 ˚c. you put in a 11.7 g ice cube at 0.00 ˚c to form a system of ice + original water. the specific heat of liquid water is 4190 j/kg•k; and the heat of fusion of water is 333 kj/kg. what is the net entropy change of the system from then until the system reaches the final (equilibrium) temperature?
Answers: 2

Physics, 23.06.2019 00:20
The brightest star in the night sky in the northern hemisphere is sirius. its distance from earth is estimated to be 8.7 light years. a light year is the distance light travels in one year. light travels at a speed of 3.00 × 108 m/s. calculate the distance from earth to sirius in miles. (1 mi = 5280 ft) g
Answers: 2
You know the right answer?
The key insight that Bohr introduced to his model of the atom was that the angular momentum of the e...
Questions




Mathematics, 12.09.2019 03:10




Physics, 12.09.2019 03:10

History, 12.09.2019 03:10

Mathematics, 12.09.2019 03:10


Mathematics, 12.09.2019 03:10




Computers and Technology, 12.09.2019 03:10