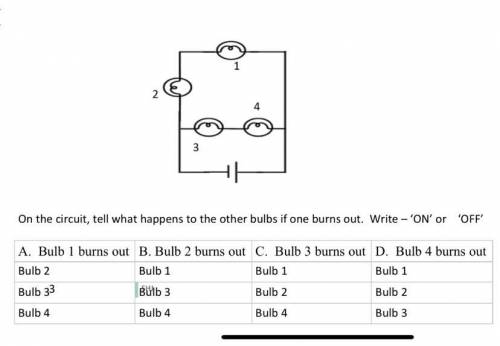
PLEASE ANSWER ASAP bbb
...

Answers: 3


Another question on Physics

Physics, 21.06.2019 16:10
In 1995 a research group led by eric cornell and carl wiemann at the university of colorado successfully cooled rubidium atoms to the 20-200 nk temperature range. assuming (incorrectly) that the rubidium atoms behave liké particles of a classical ideal gas, calculate the rms speed of a rubidium atom at a temperature of 112.0 nk. in the experiments one particular isotope of rubidium was used, rubidium-87. the molar mass of this isotope is 86.91 q/mol. tries 0/20 submit answer
Answers: 1

Physics, 22.06.2019 00:30
There are weak or strong attractive forces between atoms of liquids with a high viscosity
Answers: 3

Physics, 22.06.2019 04:50
Two technicians are discussing a resistance measurement between the can-h and can-l wires. technician a says this measurement should be done with the ignition switch in the "run" position. technician b states that a measurement of 0 ohms indicates an open in the network. which technician is correct?
Answers: 1

Physics, 22.06.2019 05:50
Acylinder with a movable piston contains 11.7 moles of a monatomic ideal gas at a pressure of 1.32×10^5 pa. the gas is initially at a temperature of 300 k. an electric heater adds 43200 j of energy into the gas while the piston moves in such a way that the pressure remains constant. cp=20.79 j k^−1 mol^−1 for a monatomic ideal gas, and that the number of gas molecules is equal to avogadro's number (6.022×10^23) times the number of moles of the gas. (a) what is the temperature of the gas after the energy is added? (b) what is the change in volume of the gas? (c) how much work is done by the gas during this process?
Answers: 3
You know the right answer?
Questions

Geography, 01.07.2019 18:30



Health, 01.07.2019 18:30

Health, 01.07.2019 18:30




Geography, 01.07.2019 18:30

Health, 01.07.2019 18:30



Health, 01.07.2019 18:30

Health, 01.07.2019 18:30





Health, 01.07.2019 18:30

Health, 01.07.2019 18:30