The equation
p1v1/t1 = p2v2/t2 is often used to study the volume of a gas at different pressu...

The equation
p1v1/t1 = p2v2/t2 is often used to study the volume of a gas at different pressures and temperatures. a scientist studying a gas found that p1v1/t1 = 44 atmospheres*liters/kelvin. if the values for standard temperature and pressure are used for the variables p1 and t1, what is the value of v1?
a) 12 liters
b) 129 liters
c) 1,200 liters
d) 12,019 liters

Answers: 1


Another question on Physics

Physics, 21.06.2019 13:30
This is how zirconium appears in the periodic table. rounded to the nearest whole number, how many electrons are in an atom of zirconium?
Answers: 2

Physics, 22.06.2019 07:00
Critical mass is the of material required to produce a chain reaction. a.) minimum amount b.) atomic mass c.) precise amount d.) maximum amount
Answers: 1

Physics, 22.06.2019 07:30
Examine the nuclear reacti why is this classified as a nuclear reaction rather than a chemical reaction? it is not balanced. a new compound is formed. a change has occurred in a nucleus. a new element has been formed.
Answers: 2

Physics, 22.06.2019 10:20
Assume that a person skiing high in the mountains at an altitude of h = 15100 ft takes in the same volume of air with each breath as she does while walking at sea level. determine the ratio of the mass of oxygen inhaled for each breath at this high altitude compared to that at sea level. assume that the air composition (i.e. % of air that is oxygen) is the same at sea level as it is at 15100 ft.
Answers: 2
You know the right answer?
Questions

History, 24.02.2021 14:00

Physics, 24.02.2021 14:00

Mathematics, 24.02.2021 14:00


Computers and Technology, 24.02.2021 14:00

Mathematics, 24.02.2021 14:00

English, 24.02.2021 14:00

Spanish, 24.02.2021 14:00

History, 24.02.2021 14:00

Mathematics, 24.02.2021 14:00


English, 24.02.2021 14:00

Mathematics, 24.02.2021 14:00


Mathematics, 24.02.2021 14:00



Physics, 24.02.2021 14:00


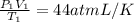
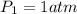
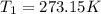
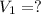
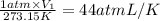
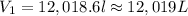
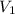 is 12.019 Liters.
is 12.019 Liters.

