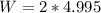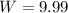Answers: 2


Another question on Physics

Physics, 22.06.2019 02:40
What happens when chlorine reacts with bromine? a. electrons move from the chlorine atoms to the bromine atoms. b. electrons move from the bromine atoms to the chlorine atoms. c. electrons are shared between the chlorine atoms and the bromine atoms. d. electrons become delocalized among the atoms.
Answers: 2


Physics, 22.06.2019 17:30
Alarge cruise ship of mass 7.00 ✕ 107 kg has a speed of 11.6 m/s at some instant. (a) what is the ship's kinetic energy at this time? (b) how much work is required to stop it? (give the work done on the ship. include the sign of the value in your answer.) (c) what is the magnitude of the constant force required to stop it as it undergoes a displacement of 3.00 km?
Answers: 2

Physics, 22.06.2019 23:40
What is the magnitude of the gravitational force acting on the earth due to the sun?
Answers: 1
You know the right answer?
The volume of an ideal gas is decreased from 5.0 l to 5.0 ml at a constant pressure of 2.0 atm. calc...
Questions

History, 30.10.2019 23:31


Mathematics, 30.10.2019 23:31



English, 30.10.2019 23:31

Mathematics, 30.10.2019 23:31

History, 30.10.2019 23:31

Mathematics, 30.10.2019 23:31


Social Studies, 30.10.2019 23:31

History, 30.10.2019 23:31


History, 30.10.2019 23:31

History, 30.10.2019 23:31

Mathematics, 30.10.2019 23:31



Advanced Placement (AP), 30.10.2019 23:31

Mathematics, 30.10.2019 23:31

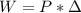
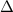 is the difference of volume.
is the difference of volume.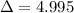 .
. 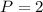 .
.