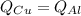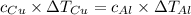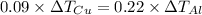Physics, 14.10.2019 09:30 krystalhurst97
Five-gram samples of copper and aluminum are at room temperature. both receive equal amounts of energy due to heat flow. the specific heat capacity of copper is 0.09 cal/g°c, and the specific heat capacity of aluminum is 0.22 cal/g°c. which of the following statements is true?
a. the temperature of each sample increases by the same amount.
b. the aluminum will get hotter than the copper.
c. the copper will get hotter than the aluminum.
d. the temperature of each sample decreases by the same amount

Answers: 1


Another question on Physics

Physics, 21.06.2019 16:30
Which of the following is not a part of a wave? question 2 options: a: frequency b: refraction c: wavelength d: amplitude (whoever answers correctly first gets brainiest! ) ~kayla
Answers: 2


Physics, 22.06.2019 16:00
What is the measure of how much matter is in an object and that can be measured using a balance? a. height b. volume c. weight d. mass
Answers: 1

Physics, 22.06.2019 19:30
Which type of energy would have nothing to do with ironing clothes? a. heat b. chemical c. electrical d. mechanical
Answers: 1
You know the right answer?
Five-gram samples of copper and aluminum are at room temperature. both receive equal amounts of ener...
Questions



Mathematics, 04.12.2020 22:10

Mathematics, 04.12.2020 22:10




History, 04.12.2020 22:10

Mathematics, 04.12.2020 22:10

Mathematics, 04.12.2020 22:10



Mathematics, 04.12.2020 22:10

Mathematics, 04.12.2020 22:10

Mathematics, 04.12.2020 22:10


English, 04.12.2020 22:10


Mathematics, 04.12.2020 22:10

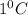
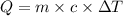
 = change in temperature
= change in temperature