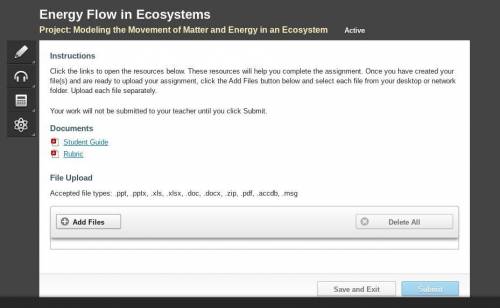
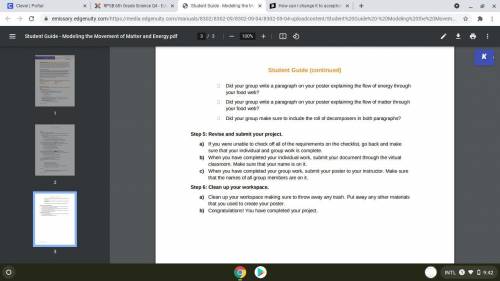
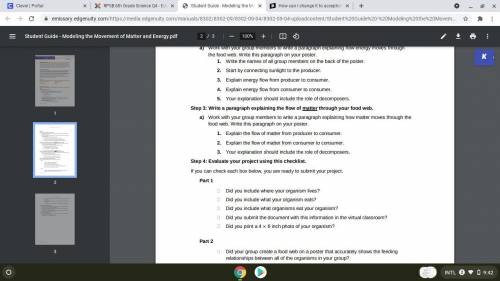
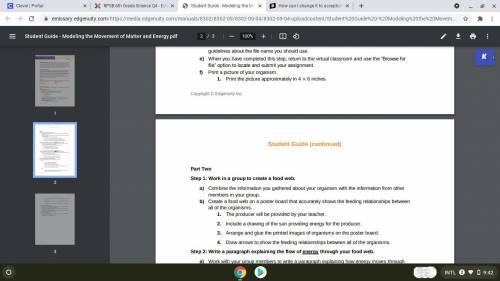
NEED HELP ASAP PLEASE HELPP
...

Answers: 3


Another question on Physics

Physics, 22.06.2019 00:00
What type of nuclear decay causes the atomic number of an element to increase by 1?
Answers: 1

Physics, 22.06.2019 11:00
1.)the isotope cobalt-60 has a nuclear mass of 59.933820 u calculate the mass defect of cobalt-60 using the following information. mass of proton: 1.007825 u mass of neutron: 1.008665 u 1 u = 931.5 mev 2.)the isotope cobalt-60 has a nuclear mass of 59.933820 u calculate the binding energy of cobalt-60 using the following information. mass of proton: 1.007825 u mass of neutron: 1.008665 u 1 u = 931.5 mev 3.)the isotope cobalt-60 has a nuclear mass of 59.933820 u calculate the binding energy per nucleon of cobalt-60 using the following information. mass of proton: 1.007825 u mass of neutron: 1.008665 u 1 u = 931.5 mev
Answers: 3

Physics, 22.06.2019 12:30
An engine undergoes a cycle as shown in the diagram. during a complete cycle the work done by the gas is 28.5×103 j and the energy transferred thermally out is 99.4×103 j. there are 1.5×1024 gas molecules and they have three active degrees of freedom. 6. (6 pts) during which process(es) is energy transferred thermally into the gas? a. 1→2 b. 2→3 c. 4→1 d. 1→2 and 4→1 e. none of the above
Answers: 3

Physics, 22.06.2019 14:00
Select for each of the following statements whether it is correct or incorrect. (a) in an isothermal expansion of an ideal gas. (b) the temperature remains constant. (b) the pressure remains constant. (c) there is work done by the gas. (d) there is heat added to the gas. (e) the change in internal energy equals zero.
Answers: 1
You know the right answer?
Questions




Mathematics, 03.07.2020 01:01


Mathematics, 03.07.2020 01:01


Mathematics, 03.07.2020 01:01







Mathematics, 03.07.2020 01:01


Mathematics, 03.07.2020 01:01


History, 03.07.2020 01:01

English, 03.07.2020 01:01