
Physics, 25.03.2021 23:40 khalaflaf9381
Consider a Carnot heat-engine cycle executed in a closed system using 0.025 kg of steam as the working fluid. It is known that the maximum absolute temperature in the cycle is twice the minimum absolute temperature, and the net work output of the cycle is 60 kJ. If the steam changes from saturated vapor to saturated liquid during heat rejection, determine the temperature of the steam during the heat rejection process. The temperature of the steam during the heat rejection process is

Answers: 2


Another question on Physics

Physics, 22.06.2019 08:00
1.what is where an object is located 2. what is how fast an objects speed is changing 3. what is how fast an object is moving
Answers: 1


Physics, 22.06.2019 17:30
Atruck driver is attempting to deliver some furniture. first, he travels 8 km east, and then he turns around and travels 3 km west. finally, he turns again and travels 13 km to his destination. what is the drivers distance
Answers: 1

Physics, 23.06.2019 00:20
You are the coordinator for a program that is going to take place at night in a rectangular amphitheater in the mountains. you will have no access to any electricity, but you must be able to illuminate the entire grounds. you know the intensity of the light from a lantern varies inversely as the square of the distance from the lantern. suppose the intensity is 90 when the distance is 5 m. a. write an equation to model the situation. b. solve for the constant of variation. c. write the equation to model the situation using the constant () of variation. d. you have been given lanterns with 40 light intensity. use your equation to solve for the distance from the lantern. e. you need to illuminate 225 km. how many meters do you need to light? f. how many lanterns will you need?
Answers: 3
You know the right answer?
Consider a Carnot heat-engine cycle executed in a closed system using 0.025 kg of steam as the worki...
Questions











Mathematics, 24.09.2019 19:40


Mathematics, 24.09.2019 19:40







Health, 24.09.2019 19:40

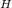 in the cycle is twice the minimum absolute temperature T
in the cycle is twice the minimum absolute temperature T in the cycle
in the cycle 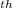 = 1 - T
= 1 - T /Q
/Q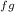 = 2400 kJ/kg
= 2400 kJ/kg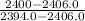 )
)

