
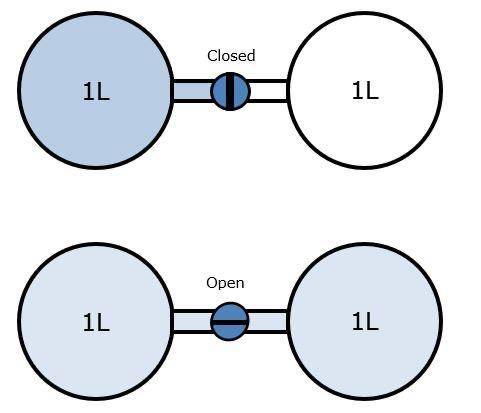
Two glass bulbs each having a volume of exactly 1L are connected by a valve. The left bulb contains a sample of nitrogen gas at a pressure of 101.325 kPa (kiloPascal), the right bulb has been emptied of gas and the pressure in the right bulb is 0 kPa.
(Use photo)
Which statement provides the BEST explanation for what happens when the valve between the bulbs is opened?
A
The volume available for the gas will double. Doubling the volume will cause the pressure and temperature to decrease.
B
The volume available for the gas will double. Doubling the volume will increases the amount of gas to keep the pressure constant.
C
The gas will expand into the empty bulb. Expansion of the gas will increase the gas pressure and decrease the gas temperature.
D
The gas will expand into the empty bulb. Expansion of the gas will increase the gas pressure and increase the gas temperature.

Answers: 3


Another question on Physics


Physics, 22.06.2019 12:30
Aboy with a mass 25 kg climbs into a small tree. he sits on a branch that is 2.o m above to the ground.what is his gravitational potential energy above the ground?
Answers: 1

Physics, 22.06.2019 14:20
How many atoms of nitrogen are in the chemical formula ni(w on
Answers: 1

Physics, 22.06.2019 22:00
Use 4 to 5 complete sentences, explain the concepts of beats and how beats are produces.
Answers: 2
You know the right answer?
Two glass bulbs each having a volume of exactly 1L are connected by a valve. The left bulb contains...
Questions


Mathematics, 10.02.2021 01:50

Mathematics, 10.02.2021 01:50

History, 10.02.2021 02:00



English, 10.02.2021 02:00


Mathematics, 10.02.2021 02:00

History, 10.02.2021 02:00


Mathematics, 10.02.2021 02:00


Mathematics, 10.02.2021 02:00

Advanced Placement (AP), 10.02.2021 02:00


Mathematics, 10.02.2021 02:00

Mathematics, 10.02.2021 02:00

Mathematics, 10.02.2021 02:00



