Physics, 18.02.2021 20:50 maddynichole2017
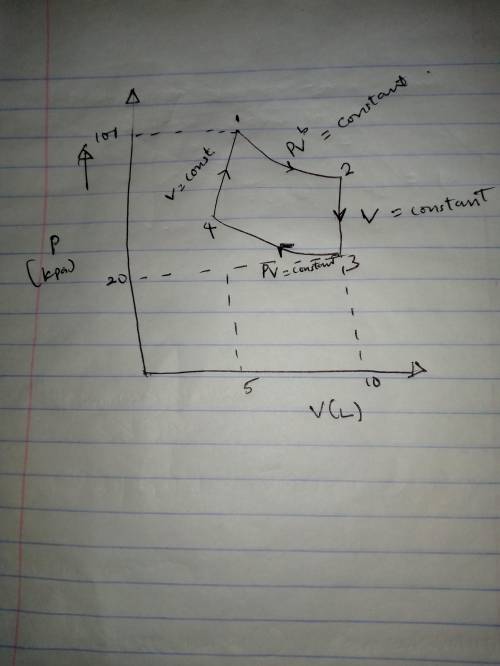
A mole of a monatomic ideal gas at point 1 (101 kPa, 5 L) is expanded adiabatically until the volume is doubled at point 2. Then it is cooled isochorically until the pressure is 20 kPa at point 3. The gas is now compressed isothermally until its volume is back to 5 L (point 4). Finally, the gas is heated isochorically to return to point 1.
a. Draw the four processes and label the points in the pV plane.
b. Calculate the work done going from 1 to 2.
c. Calculate the pressure and temperature at point 2.
d. Calculate the temperature at point 3.
e. Calculate the temperature and pressure and point 4.
f. Calculate the work done going from from 3 to 4.
g. Calculate the heat flow into the gas going from 3 to 4. g

Answers: 2


Another question on Physics

Physics, 21.06.2019 21:00
An electric current in a wire will push or pull on a permanent magnet. which statement best explains how an electric current creates an electromagnetic force on another magnet?
Answers: 2

Physics, 22.06.2019 07:20
If 2 moles of co_2 at 2l and 500k are expanded reversibly to 20l, more work can be obtained from an adiabatic process than from an isothermal process. is the above statement true or false?
Answers: 3

Physics, 22.06.2019 11:00
A2.00-m long piano wire with a mass per unit length of 12.0 g/m is under a tension of 8.00 kn. what is the frequency of the fundamental mode of vibration of this wire?
Answers: 3

Physics, 22.06.2019 13:00
Which of the following correctly describes what happens when an atomic bomb explodes? small pieces of fissionable material are joined and form a body with a mass greater than the critical mass, the relative number of neutrons escaping decreases, and a chain reaction and explosion result. large pieces of fissionable matter are brought together quickly and form a body with a mass smaller than the critical mass, the relative number of escaping neutrons increases, and a chain reaction and explosion result.
Answers: 2
You know the right answer?
A mole of a monatomic ideal gas at point 1 (101 kPa, 5 L) is expanded adiabatically until the volume...
Questions







Mathematics, 27.02.2020 19:22




History, 27.02.2020 19:23

Mathematics, 27.02.2020 19:23







Mathematics, 27.02.2020 19:23