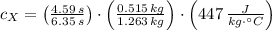Physics, 02.02.2021 05:20 batmandillon21
A small sphere of reference-grade iron with a specific heat of 447 J/kg K and a mass of 0.515 kg is suddenly immersed in a water-ice mixture. Fine thermocouple wires suspend the sphere, and the temperature is observed to change from 15 to 14C in 6.35 s. The experiment is repeated with a metallic sphere of the same diameter, but of unknown composition with a mass of 1.263 kg. If the same observed temperature change occurs in 4.59 s, what is the specific heat of the unknown material

Answers: 2


Another question on Physics

Physics, 22.06.2019 04:10
You will be galileo perform the experiment to determine if objects with different mass fall at the same, or different, rates in the air and in a vacuum. (refer to the walk-through video to locate the online lab within the online textbook).
Answers: 2

Physics, 22.06.2019 05:30
An object weighs 40n in air, weighs 20n when submerged in water and 30n when submerged in a liquid of unknown density. what is the density of the liquid?
Answers: 2

Physics, 22.06.2019 08:20
At an oceanside nuclear power plant, seawater is used as part of the cooling system. this raises the temperature of the water that is discharged back into the ocean. the amount that the water temperature is raised has a uniform distribution over the interval from 10° to 25° c. what is the standard deviation of the temperature increase?
Answers: 1

Physics, 22.06.2019 11:00
Which of the following are guidelines to follow for obtaining accurate observations
Answers: 2
You know the right answer?
A small sphere of reference-grade iron with a specific heat of 447 J/kg K and a mass of 0.515 kg is...
Questions

Physics, 31.08.2021 16:40

Arts, 31.08.2021 16:40

Mathematics, 31.08.2021 16:40


Computers and Technology, 31.08.2021 16:40

Mathematics, 31.08.2021 16:40

Mathematics, 31.08.2021 16:40

English, 31.08.2021 16:40


Computers and Technology, 31.08.2021 16:40









History, 31.08.2021 16:40

 ), measured in watts, that is, joules per second, by the following formula:
), measured in watts, that is, joules per second, by the following formula: (1)
(1) 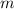 - Mass of the sphere, measured in kilograms.
- Mass of the sphere, measured in kilograms. - Specific heat of the material, measured in joules per kilogram-degree Celsius.
- Specific heat of the material, measured in joules per kilogram-degree Celsius.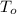 ,
, 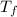 - Initial and final temperatures of the sphere, measured in degrees Celsius.
- Initial and final temperatures of the sphere, measured in degrees Celsius. - Time, measured in seconds.
- Time, measured in seconds.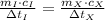 (2)
(2)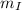 ,
, 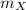 - Masses of the iron and unknown spheres, measured in kilograms.
- Masses of the iron and unknown spheres, measured in kilograms.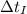 ,
, 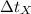 - Times of the iron and unknown spheres, measured in seconds.
- Times of the iron and unknown spheres, measured in seconds.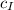 ,
, 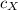 - Specific heats of the iron and unknown materials, measured in joules per kilogram-degree Celsius.
- Specific heats of the iron and unknown materials, measured in joules per kilogram-degree Celsius.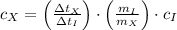
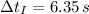 ,
,  ,
, 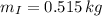 ,
, 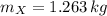 and
and 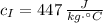 , then the specific heat of the unknown material is:
, then the specific heat of the unknown material is: