
The annual production of Cl2 is about 45 millions tons per year. Assume that a typical plant is operational 90 % of the year. The operating voltage of a cell is 3.4 V (considerably higher than the equilibrium voltage)
(a) Write down the half-cell reaction for the oxidation chloride to form chlorine.
(b) Determine the total current worldwide needed to gener- ate the global supply of Cl2.
(c) Calculate the electrical power needed to produce the global supply of chlorine using electrolysis.

Answers: 2


Another question on Physics

Physics, 22.06.2019 00:30
Asap time is ! best answer gets compose at least one well-developed paragraph on the following: define the term concurrent powers, and give an example of a concurrent power of government.
Answers: 1

Physics, 22.06.2019 19:20
Which best describes the difference between internal and thermal energy?
Answers: 1

Physics, 22.06.2019 23:00
The gun from the crime scene shows tool marks where the serial numbers are ground off. there are also tool marks on the bullets left behind. what information can the tool marks on the bullets and gun provide about the crime? what type of evidence does this information provide, individual or class?
Answers: 1

Physics, 23.06.2019 01:30
)consider two positively charged particles, one of charge q0 (particle 0) fixed at the origin, and another of charge q1 (particle 1) fixed on the y-axis at (0,d1,0). what is the net force f⃗ on particle 0 due to particle 1?
Answers: 2
You know the right answer?
The annual production of Cl2 is about 45 millions tons per year. Assume that a typical plant is oper...
Questions




Mathematics, 24.11.2020 04:20


Mathematics, 24.11.2020 04:20

Mathematics, 24.11.2020 04:20



Mathematics, 24.11.2020 04:20


Mathematics, 24.11.2020 04:20


Computers and Technology, 24.11.2020 04:20

Mathematics, 24.11.2020 04:20



Mathematics, 24.11.2020 04:20

English, 24.11.2020 04:20

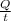 =
= 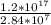 = 4.2 * 10^9 amperes
= 4.2 * 10^9 amperes


