
A 0.4000 kg sample of methanol at 16.0ºC is mixed with 0.4000 kg of water at 85.0ºC. Assuming no heat loss to the surroundings, what is the final temperature of the mixture? The specific heat of methanol is 2450 J/kg•ºC, the specific heat capacity of liquid water is 4180 J/kg⋅°C

Answers: 3


Another question on Physics

Physics, 21.06.2019 16:30
Aball bearing at 220.0 °c is dropped into a cup containing 250.0 g of water at 20.0 °c. the water and ball bearing come to a temperature of 30.0 °c. what is the heat capacity of the ball bearing in j/°c? (hint: how are the algebraic signs of q related for the ball bearing and the water? )
Answers: 1

Physics, 21.06.2019 19:30
The us government wants to allocate billions of dollars in the next 10 years to assure our future energy security. the funds will be spread among a variety of possible energy resources. where do you think the government should put the greatest support: solar energy, wind energy, clean coal, oil exploration, gas exploration, or a combination of sources? are there other efforts that should be explored? support your position with cited information for both questions.
Answers: 2

Physics, 22.06.2019 08:30
The coefficient of friction is a number that represents the resistance to sliding between two in contact with one another.
Answers: 2

You know the right answer?
A 0.4000 kg sample of methanol at 16.0ºC is mixed with 0.4000 kg of water at 85.0ºC. Assuming no hea...
Questions


Mathematics, 22.04.2021 23:30




Mathematics, 22.04.2021 23:30


Mathematics, 22.04.2021 23:30


Biology, 22.04.2021 23:30


English, 22.04.2021 23:30


Mathematics, 22.04.2021 23:30

Social Studies, 22.04.2021 23:30


Mathematics, 22.04.2021 23:30

Health, 22.04.2021 23:30

History, 22.04.2021 23:30

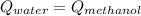
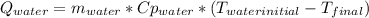
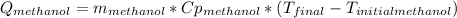
![0.4*4180*(85-T_{final})=0.4*2450*(T_{final}-16)\\142120-1672*T_{final}=980*T_{final}-15680\\157800=2652*T_{final}\\T_{final}=59.5[C]](/tpl/images/1031/5237/e4a4e.png)



