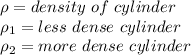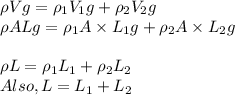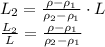Physics, 12.01.2021 18:00 live4dramaoy0yf9
A less-dense liquid of density rho1 floats on top of a more-dense liquid of density rho2. A uniform cylinder of length l and density rho, with rho1

Answers: 2


Another question on Physics

Physics, 22.06.2019 09:30
Which are advantages of renewable resources? check all that apply. renewable energy supplies are completely reliable everywhere. some renewable resources will never be used up. little or no waste is produced by renewable resource plants. electricity can be generated in large quantities. many renewable energy facilities have lower operating costs.
Answers: 1

Physics, 22.06.2019 23:20
Acharge q experiences no net force at a particular point in space. which of the following situations described below must always be true? -there are no other charges nearby. -if there are other charges nearby, they must all have the same sign as q. -if there are other charges nearby, they must all have the opposite sign of q. -if there are other charges nearby, the total positive charge must equal the total negative charge. -none of the above
Answers: 2

Physics, 23.06.2019 01:30
The field lines around one end of a bar magnet are shown below based on the diagram, what can you conclude about the pole of the magnet? a.is a south pole because the field hnes spread out from this end b.a north pole because the field lines spread out from this end c.a nouth pole because the field lines enter the magnet at this end d.is a north pole because the field lines enter the magnet at this end
Answers: 1

Physics, 23.06.2019 08:00
Use henry's law and the solubilities given below to calculate the total volume of nitrogen and oxygen gas that should bubble out of 1.7 l of water upon warming from 25 ˚c to 50 ˚c. assume that the water is initially saturated with nitrogen and oxygen gas at 25 ˚c and a total pressure of 1.0 atm. assume that the gas bubbles out at a temperature of 50 ˚c. the solubility of oxygen gas at 50 ˚c is 27.8 mg/l at an oxygen pressure of 1.00 atm. the solubility of nitrogen gas at 50 ˚c is 14.6 mg/l at a nitrogen pressure of 1.00 atm. assume that the air above the water contains an oxygen partial pressure of 0.21 atm and a nitrogen partial pressure of 0.78 atm.
Answers: 2
You know the right answer?
A less-dense liquid of density rho1 floats on top of a more-dense liquid of density rho2. A uniform...
Questions

English, 15.04.2020 06:35



History, 15.04.2020 06:35


History, 15.04.2020 06:36


Mathematics, 15.04.2020 06:36

Chemistry, 15.04.2020 06:36

Mathematics, 15.04.2020 06:36


Biology, 15.04.2020 06:36



Chemistry, 15.04.2020 06:36





Chemistry, 15.04.2020 06:36