
A 0.1000 kg brass block at 100.0°C is placed in 0.2000 kg of water at
20.0°C. The specific heat of brass is 376 J/kg•°C, the specific heat
capacity of liquid water is 4180 J/kg-°C. Assuming no heat loss to the
surroundings, what is the final temperature of the mixture? *
Pls help I’ll do anything

Answers: 2


Another question on Physics


Physics, 22.06.2019 12:20
Which lists the pairs of plates in order from least to greatest in terms of the work done to move the electron?
Answers: 2

Physics, 22.06.2019 15:20
Arigid tank is divided into two equal parts by a partition. one part of the tank contains 3 kg of compressed liquid water at 400 kpa and 60°c while the other part is evacuated. the partition is now removed, and the water expands to fill the entire tank. determine the entropy change of water during this process, if the final pressure in the tank is 40 kpa. use steam tables.
Answers: 3

Physics, 22.06.2019 18:30
Aballoon is rising vertically upwards at a velocity of 10m/s. when it is at a height of 45m from the ground, a parachute bails out from it. after 3s he opens his parachute and decelerates ata a constant rate 5m/s.when. (a) what was the height of the parachutist above the ground when he opened his parachute? (b)how far is the parachutist from the balloon at t=3s? (c)with what velocity does the parachutist hit the ground? (d)after how long does the parachutist hit the ground after his exist from the balloon?
Answers: 3
You know the right answer?
A 0.1000 kg brass block at 100.0°C is placed in 0.2000 kg of water at
20.0°C. The specific heat of...
Questions


Mathematics, 28.06.2019 12:00



Computers and Technology, 28.06.2019 12:00


Mathematics, 28.06.2019 12:00


Mathematics, 28.06.2019 12:00


Mathematics, 28.06.2019 12:00

Chemistry, 28.06.2019 12:00


Chemistry, 28.06.2019 12:00


English, 28.06.2019 12:00

English, 28.06.2019 12:00



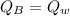
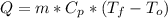
![0.1*376*(100-T_{f})=0.2*4180*(T_{f}-20)\\3760-37.6*T_{f}=836*T_{f}-16720\\3760+16720=836*T_{f}+37.6*T_{f}\\20480=873.6*T_{f}\\T_{f}=23.44[C]](/tpl/images/1028/6079/778c5.png)


