

Answers: 2


Another question on Physics


Physics, 22.06.2019 12:30
Apositive charge moves in the direction of an electric field. which of the following statements are true? check all that apply. check all that apply. 1.the potential energy associated with the charge decreases. 2. the electric field does positive work on the charge. 3. the electric field does negative work on the charge. 4. the potential energy associated with the charge increases. 5. the electric field does not do any work on the charge. 6. the amount of work done on the charge cannot be determined without additional information.
Answers: 1

Physics, 22.06.2019 17:40
You throw a baseball directly upward at time =0 at an initial speed of 14.9 m/s. what is the maximum height the ball reaches above where it leaves your hand? ignore air resistance and take =9.80 m/s2.
Answers: 1

Physics, 22.06.2019 18:00
Wind and moving water provide energy. question 1 options: chemical mechanical thermal none of the above
Answers: 1
You know the right answer?
A (10.0+A) g ice cube at -15.0oC is placed in (125 B) g of water at 48.0oC. Find the final temperatu...
Questions



Mathematics, 24.08.2019 22:30

Advanced Placement (AP), 24.08.2019 22:30


Social Studies, 24.08.2019 22:30

Mathematics, 24.08.2019 22:30

Social Studies, 24.08.2019 22:30

Mathematics, 24.08.2019 22:30

Mathematics, 24.08.2019 22:30


Mathematics, 24.08.2019 22:30

Mathematics, 24.08.2019 22:30


English, 24.08.2019 22:30

Mathematics, 24.08.2019 22:30




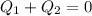
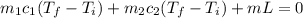
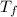 is final temperature
is final temperature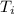 is initial temperature
is initial temperature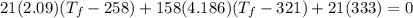
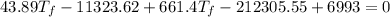
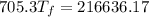
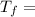 307.15K
307.15K

