Use the image below to answer the question.
What does the arrow 'B' represent?
Question 7 opt...

Physics, 02.12.2020 01:40 xxxamslashxxx9
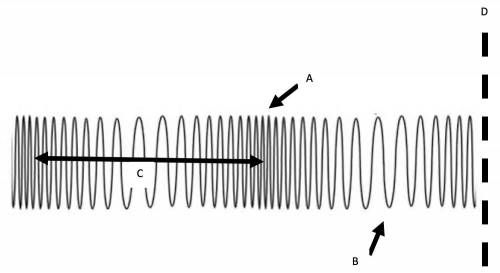
Use the image below to answer the question.
What does the arrow 'B' represent?
Question 7 options:
wavelength
refraction
frequency
compression

Answers: 2


Another question on Physics

Physics, 21.06.2019 23:30
Part a determine the magnitude of the x component of f using scalar notation. fx f x = nothing lb request answer part b determine the magnitude of the y component of f using scalar notation. fy f y = nothing lb request answer part c determine the magnitude of the z component of f using scalar notation. fz f z = nothing lb request answer provide feedback figure1 of 1a force vector acting on a ring attached to the ground is shown in the xyz space together with its x, y, and z components lying on the corresponding positive axes. the ring is located at the origin. force f is located in the first octant. f makes an angle of 60 degrees with its x component and an angle of 45 degrees with its y component. a force vector acting on a ring attached to the ground is shown in the xyz space together with its x, y, and z components lying on the corresponding positive axes. the ring is located at the origin. force f is located in the first octant. f makes an angle of 60 degrees with its x component and an angle of 45 degrees with its y component.
Answers: 2

Physics, 22.06.2019 10:00
(a) calculate the number of electrons in a small, electrically neutral silver pin that has a mass of 10.0 g. silver has 47 electrons per atom, and its molar mass is 107.87 g/mol. (b) imagine adding electrons to the pin until the negative charge has the very large value 1.00 mc. how many electrons are added for every 109 electrons already present
Answers: 3

Physics, 22.06.2019 11:00
Which sound characteristic is not affected by the relative motion of an object
Answers: 2

Physics, 22.06.2019 11:20
Wave functions describe orbitals in a hydrogen atom. each function is characterized by 3 quantum numbers: n, l, and ml. if the value of n = 2: the quantum number l can have values from to . the total number of orbitals possible at the n = 2 energy level is .
Answers: 3
You know the right answer?
Questions




History, 02.10.2019 09:10


Mathematics, 02.10.2019 09:10

Social Studies, 02.10.2019 09:10

Social Studies, 02.10.2019 09:10

English, 02.10.2019 09:10

Mathematics, 02.10.2019 09:10


Mathematics, 02.10.2019 09:10

Business, 02.10.2019 09:10

Computers and Technology, 02.10.2019 09:10




Chemistry, 02.10.2019 09:10




