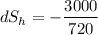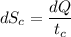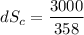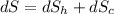When an aluminum bar is connected between a hot reservoir at 720 K and a cold reservoir at 358 K, 3.00 kJ of energy is transferred by heat from the hot reservoir to the cold reservoir. (a) In this irreversible process, calculate the change in entropy of the hot reservoir. J/K
(b) In this irreversible process, calculate the change in entropy of the cold reservoir.
J/K
(c) In this irreversible process, calculate the change in entropy of the Universe, neglecting any change in entropy of the aluminum rod.
J/K
(d) Mathematically, why did the result for the Universe in part (c) have to be positive?

Answers: 3


Another question on Physics

Physics, 22.06.2019 04:20
When considering gravity acceleration and the force of acceleration, what must be true? a. the direction of acceleration must be perpendicular to the direction of the force. b. the direction of the force and the direction of acceleration must be the same as each other. c. the direction of the force and the direction of acceleration must be opposite of each other. d. the mass of the body must be the same as the acceleration of the body.
Answers: 1

Physics, 22.06.2019 13:50
Observations show that interstellar clouds can have almost any shape and
Answers: 1


Physics, 22.06.2019 18:00
Consider an ideal gas at 27.0 degrees celsius and 1.00 atmosphere pressure. imagine the molecules to be uniformly spaced, with each molecule at the center of a small cube. what is the length l of an edge of each small cube if adjacent cubes touch but don't overlap?
Answers: 2
You know the right answer?
When an aluminum bar is connected between a hot reservoir at 720 K and a cold reservoir at 358 K, 3....
Questions




Mathematics, 13.07.2019 17:50



Social Studies, 13.07.2019 17:50

Social Studies, 13.07.2019 17:50

Business, 13.07.2019 17:50

English, 13.07.2019 17:50

English, 13.07.2019 17:50

Mathematics, 13.07.2019 17:50





Biology, 13.07.2019 17:50


Biology, 13.07.2019 17:50

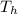 = 720 K
= 720 K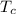 = 358 K
= 358 K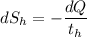 (the negative sign shows the loss of heat)
(the negative sign shows the loss of heat)