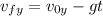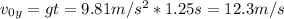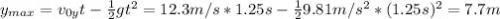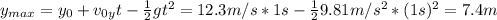Answers: 2


Another question on Physics

Physics, 22.06.2019 05:50
Acylinder with a movable piston contains 11.7 moles of a monatomic ideal gas at a pressure of 1.32×10^5 pa. the gas is initially at a temperature of 300 k. an electric heater adds 43200 j of energy into the gas while the piston moves in such a way that the pressure remains constant. cp=20.79 j k^−1 mol^−1 for a monatomic ideal gas, and that the number of gas molecules is equal to avogadro's number (6.022×10^23) times the number of moles of the gas. (a) what is the temperature of the gas after the energy is added? (b) what is the change in volume of the gas? (c) how much work is done by the gas during this process?
Answers: 3

Physics, 22.06.2019 11:00
Although longitudinal waves can travel through all media types what is the disadvantage of this type of wave transmission? a) he efficiency of he wave transmission is greatly diminished as the media becomes less dense b) the oscillation of one set of particles has a huge impact on neighboring particles c) particles become too close together as they bump into each other making it more likely to hit other particles down the line
Answers: 2

Physics, 22.06.2019 14:30
Which of these is constant for all types of electromagnetic radiation in a vacuum? select one: a. wavelength b. frequency c. photon energy d. amplitude e. velocity
Answers: 3

Physics, 22.06.2019 17:40
A15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 25°c to 175°c. what is the specific heat capacity of iron?
Answers: 1
You know the right answer?
Se lanza una pelota y regresa al punto de partida 2.5 s después ¿Que altura máxima alcanzara? ¿a que...
Questions




Computers and Technology, 28.09.2021 19:10


English, 28.09.2021 19:10




Chemistry, 28.09.2021 19:10

Mathematics, 28.09.2021 19:10

Mathematics, 28.09.2021 19:10


Mathematics, 28.09.2021 19:10

English, 28.09.2021 19:10




History, 28.09.2021 19:20

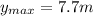
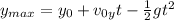 (1)
(1)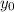 : es la altura inicial = 0
: es la altura inicial = 0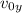 : es la velocidad inicial en y
: es la velocidad inicial en y