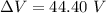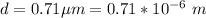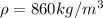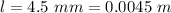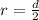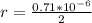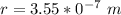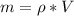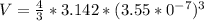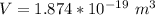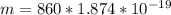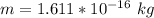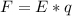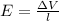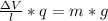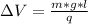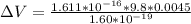Physics, 14.10.2020 01:01 battlemarshmell
In the early 1900s, Robert Millikan used small charged droplets of oil, suspended in an electric field, to make the first quantitative measurements of the electron’s charge. A 0.71-um-diameter droplet of oil, having a charge of , is suspended in midair between two horizontal plates of a parallel-plate capacitor. The upward electric force on the droplet is exactly balanced by the downward force of gravity. The oil has a density of 860 kg/m^3 , and the capacitor plates are 4.5 mm apart.
Part A
What must the potential difference between the plates be to hold the droplet in equilibrium?
Express your answer to two significant figures and include the appropriate units
ΔV=

Answers: 1


Another question on Physics



Physics, 22.06.2019 18:00
Gabby calls her cousin who lives in a different state and tells her to turn her radio to channel 98.7 so they can listen to their favorite song that is playing. her cousin turns her radio to 98.7, but does not hear the same song gabby hears. which most likely explains why?
Answers: 1

Physics, 22.06.2019 21:20
An electron is ejected into a horizontal uniform e⃗ field at a parallel horizontal velocity of v0. assume the electron's initial position x0, initial velocity v0, time t, magnitude of electric field e, electron's mass m, and the magnitude of the electron's charge |e|. ignore the force that earth exerts on the electron. assume the e⃗ field is in the same direction as the initial velocity. part a define the equation for the electron's velocity. express your answer in terms of the variables v0, |e|, t, e, and m.
Answers: 3
You know the right answer?
In the early 1900s, Robert Millikan used small charged droplets of oil, suspended in an electric fie...
Questions






History, 21.05.2020 04:11



Mathematics, 21.05.2020 04:11

Chemistry, 21.05.2020 04:12


Mathematics, 21.05.2020 04:12




Biology, 21.05.2020 04:12



Mathematics, 21.05.2020 04:57

Mathematics, 21.05.2020 04:57