
Physics, 08.10.2020 01:01 ashtyndowdle
2. The visible region of the hydrogen spectrum results from relaxation of electrons from excited states to energy level 2 (n1). Use the Rydberg equation and your measured wavelengths to determine the energy transitions associated with each of your observed wavelengths for hydrogen. In other words, calculate the excited state energy level (n2) for each of your observed wavelengths for hydrogen. n has integer values; so, calculate it first with appropriate significant digits, then round it to an integer. Use the key to show your work for at least one calculation. Must show energy levels for each hydrogen wavelength.

Answers: 2


Another question on Physics

Physics, 22.06.2019 04:00
When the force acting on an object points at least partially in the direction of the motion the work done is considered to be negative
Answers: 2

Physics, 22.06.2019 06:10
Africtionless piston-cylinder contains 50 l of saturated liquid r-134a. the piston has a mass and an area resulting in an applied pressure of 500 kpa on the refrigerant. the refrigerant is now heated until its temperature rises to 70∘c. calculate the work (in kj) done during this process.
Answers: 3

Physics, 22.06.2019 13:50
9.98 kg of r-134a at 300 kpa fills a rigid container whose volume is 14 l. determine the temperature and total enthalpy in the container. the container is now heated until the pressure is 600 kpa. determine the temperature and total enthalpy when the heating is completed. use data from the steam tables.
Answers: 1

Physics, 23.06.2019 00:20
The brightest star in the night sky in the northern hemisphere is sirius. its distance from earth is estimated to be 8.7 light years. a light year is the distance light travels in one year. light travels at a speed of 3.00 × 108 m/s. calculate the distance from earth to sirius in miles. (1 mi = 5280 ft) g
Answers: 2
You know the right answer?
2. The visible region of the hydrogen spectrum results from relaxation of electrons from excited sta...
Questions


English, 12.10.2019 02:10

Business, 12.10.2019 02:10

History, 12.10.2019 02:10



English, 12.10.2019 02:10

Mathematics, 12.10.2019 02:10


Computers and Technology, 12.10.2019 02:10

Mathematics, 12.10.2019 02:10



Mathematics, 12.10.2019 02:10


Mathematics, 12.10.2019 02:10

History, 12.10.2019 02:10



Computers and Technology, 12.10.2019 02:10

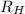 (1/2² - 1 / n²)
(1/2² - 1 / n²)


